Pushpa Telugu Movie Review, Rating
పుష్ప తెలుగు సినిమా రివ్యూ ,రేటింగ్
-
UTS App: Book General and Platform Tickets Instantly Using Your Phone
-
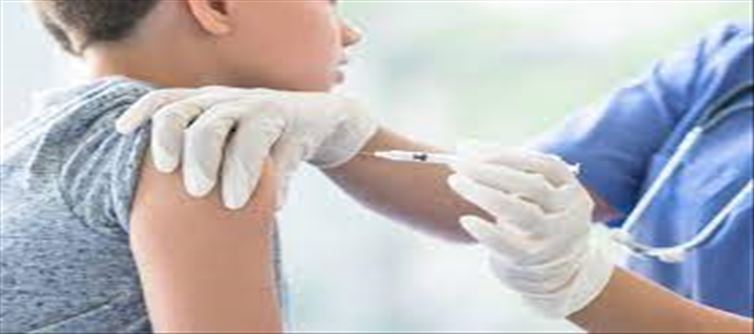
Insurance Tips: 5 Things You Must Include in Your Health Insurance Plan
-
Revitalize Your Skin: Five Natural Face Packs for Post-Festive Detox
-
Exam: XAT 2026 Admit Card News📄
-
India Aviation News: New Airlines Approved by Government
-
Uttar Pradesh Metro Expansion News Highlights
-
Vaibhav Suryavanshi & Vijay Hazare Trophy Highlights
-
Effective Yoga Practices to Combat Air Pollution Effects on Lungs
-
Essential Parenting Insights: Grandparents’ Role in Child Development
-
Rashmika Mandanna Transforms into a Tribal Girl for Her Latest Role
-
Delicious Buckwheat Barfi Recipe: A Healthy Twist on a Classic Indian Sweet
-
Essential Co-Parenting Tips for Divorced Parents
-
Understanding the Impact of the Sun in the Second House
-
Can Apples Help Lower Cholesterol Levels?
-
Five Energizing Morning Drinks for Better Digestion
-
Jana Nayagan Set for Massive Pongal Box Office Collection; Fans Excited by Twitter Update
-
Allu Arjun Joins Lokesh Kanagaraj’s Long-Time Dream Project; High-Quality Action in Store
-
Thalapathy Vijay Sings in Style: Third Song from Jana Nayagan Released
-
Jailer 2 Update: Shah Rukh Khan to Play a Key Character Named Gaurav
-
Lokesh Kanagaraj Meets Allu Arjun, Next Project Already in the Works
-
Sira Review: Vikram Prabhu Impresses with a Powerful Performance
-
Jana Nayagan’s Advance Booking & Box Office Preview: How Much It Has Collected So Far
-
DMK to Contest in 8 Constituencies in Madurai;
-
Parasakthi Caught in Censorship Controversy
-
Sevala Kaala Being Made Against the Backdrop of Madurai
-
Third Song from Jan Nayagan, Sung by Vijay, to Be Released Tomorrow
-
Strength of the Majority Lies in Standing With Minorities So They Can Live Without Fear
-
Chief Minister M.K. Stalin to Visit Kallakurichi Tomorrow to Distribute Welfare Assistance to Two Lakh Beneficiaries
-
Jan Nayagan: Actor Vijay Sings the Song “Chella Magale” – Third Single from the Film Released
-
Restrictions Imposed on Yellow and Red-Coloured Items at Jan Nayagan Audio Launch Event
-
Attacks on Minorities Are Unacceptable: Chief Minister M.K. Stalin Condemns Violence in Chhattisgarh and Assam
-
Minister E.V. Velu Reviews Preparations for Agricultural Exhibition to Be Inaugurated by the Chief Minister in Tiruvannamalai on the 27th
-
Essential Skincare Tips for Expecting Mothers: What to Avoid
-
Smart TV Indicator Light: What That Small Blinking Light Really Means and How It Can Extend Your TV’s Life
-
Voter List 2025: How to Check if Your Name is on the New Voter List Using Your Phone
-
How to Change Your Address on Your Passport Online Using Your Phone
-
Personal Loan Guide: 5 Key Questions to Ask Before Choosing the Best and Cheapest Loan
-
Is Your Ration Card Getting Damaged? Here’s How to Get an ATM-like PVC Card
-
Essential Tips for Healthier Tea: What to Avoid
-
Does Pressing the Cancel Button After Withdrawing Cash Really Keep Your PIN Safe?
-
Aadhaar-PAN Linking: How to Link from Home & What Happens if You Don’t
-
Indian Railways Fare Hike: How Much Extra Will You Pay from December 26? 🚆💸
-
RBI Lateral Recruitment 2025: B.Tech Graduates Can Apply! 💼🏦
-
E-Passport Launched in India: How to Apply, Key Features, and Benefits of the Chip-Based Passport
-
Essential Vastu Tips for Enhancing the Tulsi Plant’s Energy
-
IIMC Non-Teaching Vacancy 2025: Graduates Can Apply! 📝🎓
-
Chief Minister Women Employment Scheme: Last Chance to Claim ₹10,000! 💸👩💼
-
Aadhaar Update from Home: Easy Step-by-Step Guide 🆔💻
-
NEET UG 2026: Why Coaching Has Become Essential for a Top Score 🩺📚
-
Fake QR Codes Can Hijack Your WhatsApp: How This New Scam Works and How to Stay Safe
-
Understanding the Best Sleeping Direction for Health and Well-Being
-
PM Awas Yojana Gramin: How to Apply, Check List & Eligibility 🏠🌾
-
Budget 2026: Will Farmers’ Income Get a Boost? Focus on PM-Kisan Scheme 🌾💰
-
Income Tax Refund Delays Explained: Form 16 Mismatch May Be the Culprit 🧾💸
-
December 25,2025 Daily Panchagam
-
December 25,2025 Daily Horoscope(Astrology)
-
Which famous people were born on 25 December in India?
-
Which cricketer was born on 25 December?
-
Christmas wishes and quotes
-
Whose birthday is on 25 December in India?
-
What are the top 10 most popular Christmas decorations?
-
Which freedom fighter died on 25 December?
-
What are popular Christmas colors for 2025?
-
Who is the sun god born on December 25?
-
What is on 25 December in Hindu?
-
What is the theme for Christmas 2025?
-
U.S. Public Charge Rule: What It Means for Green Card Applicants
-
Enhancing Home Energy: The Power of Positive Paintings
-
What happened on 25 December in Indian history?
-
5 Christmas Movies to Stream on JioHotstar for Cozy Festivities and Film Nights
-
Why is Christmas really celebrated?
-
Why is 25 December called Big day?
-
Healthy Oats Halwa Recipe: A Nutritious Twist on a Classic Dessert
-
Kawasaki W230 Teased — Could an India Launch Be Next?
-
Why is Santa red and white?
-
Who was born on 25 December?
-
Tata Magic Iris Wants to Be the Mahindra Bolero — But Did It Succeed?
-
Discover the Health Benefits of Boiled Peanuts
-
Hyundai Motor to Recall 51,587 Vehicles in the US Over Potential Fire Risk
-
Why You Should Visit Mumbai’s Afghan Church This Christmas
-
What's the connection between Jesus and Santa?
-
Why is December 25 so special?
-
How Mumbai Makes All Feel at Home: A Quiet Tale of Social Security
-
Merry Christmas 2025: Heartfelt Wishes, Messages, Greetings & Quotes to Share on X’Mas Day
-
Christmas 2025: Ananya Panday, Khushi Kapoor & Other Bollywood Celebs-Inspired Red Looks to Steal This Season
-
Cristiano Ronaldo Now Owns Two Ultra-Luxury Villas in Saudi Arabia’s Red Sea:
-
The Benefits of Makhana: A Superfood for Children's Health
-
What is the actual history of Christmas?
-
Tu Meri Main Tera Main Tera Tu Meri Advance Booking Report
-
Delicious and Nutritious Dry Fruit Panjiri Recipe: A Wholesome Traditional Treat
-
What special day is on 25 December?
-
Merry Christmas 2025: Date, Celebration, and Significance
-
🌾 “A Voice for All Indian Farmers from Tamil Nadu” — M.K. Stalin
-
The Tamil news about contract nurses being made permanent as announced by Minister Ma. Subramanian:
-
🏏 “I’m an Off Spinner I Bowled to Kalaignar Got Simbu’s Wicket!” — Emotional Stalin on Cricket
-
“Thaai Kizhavi” Teaser Released — Radhika Sarathkumar’s Powerful New Look Impresses Audiences
-
Political Speeches Banned at Jana Nayagan Music Launch; Malaysian Police Issue Warning
-
Chief Minister's Free Electricity Scheme: ₹17,000 Transferred to Beneficiaries’ Accounts in Rajasthan
-
Silver Purity Test: How to Spot Fake Silver Easily 🪙✨
-
Indian Railways to Hike Train Fares from December 26, 2025 🚆💸
-
CBSE Vacancy 2025: Apply Online for 124 Posts Before December 27! 📚✏️
-
Instagram vs YouTube: Long-Form Videos Coming Soon? 🎥📱
-
Widow Pension Scheme: Bihar’s Indira Gandhi National Widow Pension Scheme
-
Delicious Semolina Dhokla Sandwich Recipe for a Joyful Breakfast
-
Home Loan Interest Rates Drop: CIBIL Score Now Key to Better Rates 🏡💳
-
Income Tax Alerts Coming Late at Night? Act Before Dec 31 to Secure Your Refund! ⏰💰
-
Tech Explained: What Is the Difference Between MP3 and MP4?
-
Digital Era of Tax Scrutiny: Income Tax Investigations Going Fully Online from 2026 💻
-
ICICI Bank Credit Card Users Alert: Charges Revised on Gaming, Wallet & Transport Payments from 2026 💳
-
Loans & Tax Savings: How to Make the Most of Home, Education, Car, and Personal Loans 💰
-
Become Debt-Free Sooner: 3 Smart Ways to Pay Off Your Loan Early 💸
-
Post Office Time Deposit Scheme: Invest Once, Earn ₹2 Lakh in Interest! 💰
-
Delightful Mishri Mawa Recipe: A Sweet Treat for Every Occasion
-
Indian Railways Updates Online Ticket Booking Rules
-
5 Settings Are the “Enemies” of Your Phone’s Battery
-
JEE Main vs JEE Advanced: Key Differences Every Aspirant Must Know 🎓
-
Income Tax Department Alerts: Why You’re Receiving Emails About Deductions 💡
-
SBI SCO Recruitment 2025: Apply Online Before January 5! 🏦
-
Delicious Aloo Masala Kachori Recipe to Satisfy Your Cravings
-
PM Surya Ghar: How to Apply Online for the Free Electricity Scheme
-
CBDT NUDGE Campaign 2025-26: Correct Your ITR Errors Before Dec 31! 💰
-
Bajra Roti Health Benefits: Why This Traditional Indian Bread Is a Superfood
-
RRB Group D Recruitment 2026: Notification Released for Millions! 🚂
-
Finance Ministry Jobs 2025: Young Professionals & Consultants Wanted! 💼
-
Chief Minister Stalin’s Christmas Message: Referencing Thirukkural, Unity, Love and Secular Values
-
From Politics at 14 to Managing Tension: CM Stalin Opens Up
-
Walnut Remedy to Keep Eyes Strong Even in Old Age
-
DMK Alliance Leaders Protest at Madavakkam Against Union Government: Veeramani, Vaiko, Thirumavalavan Take Part
-
WiFi Tips: Is Your WiFi Not Reaching Other Rooms?
-
RRB Group D Recruitment 2025: 22,000 Railway Posts Coming Soon! 🚂
-
NLC Apprentice Recruitment 2025: B.Tech & Diploma Holders Can Apply! ⚡
-
Science Behind Dreams and Nightmares: What Happens When Your Brain Is Sleeping
-
Over $1 Billion Clear: Avatar Obliterates Star Wars’ Lifetime Record
-
UPPSC Recruitment 2025: Medical Officers, Dental Surgeons & More – Apply Now! 🏥
-
5 Low-Maintenance Balcony Plants Ideal for Every Small Apartment
-
RRB Group D Recruitment 2025: 22,000 Vacancies Announced – Don’t Miss Out! 🚆
-
5 Morning Yoga Asanas That Boost Your Gut Health Naturally in Winter
-
What Happens If You Take a Vehicle Without a PUC Certificate to a Petrol Pump?
-
Sleeping With Wet Hair: Is This Habit Secretly Damaging Your Hair?
-
SSC Stenographer Recruitment 2025: 6 Major Departments Hiring Now!
-
AIIMS Patna Recruitment 2025: Vacancies Open for Senior Resident Positions in 28 Departments
-
Minor PAN Card: When Is a Child’s PAN Card Needed, and How Can You Apply for One?
-
From Stranger Things to Omega-Level Mutant: Sadie Sink’s MCU Shockwave
-
How to Prepare Your Body Before Egg Freezing
-
Bihar Police SI Exam 2026: Preliminary Exam Scheduled for January 18 & 21
-
🎄 10 Best Christmas Card Design DIY Ideas for School Friends, Teachers & Principal ✂️✨
-
IOCL Recruitment 2025: Over 350 Vacancies Open for Junior Engineers and Quality Control Analysts
-
Credit Card vs. Personal Loan: Advantages, Disadvantages, and the Smart Choice When You’re Short on Money
-
Why Your Skincare Routine Is Ruining Your Skin: 6 Everyday Mistakes No One Warns You About
-
🎄 Christmas Drawing Ideas 2025: 10+ Christmas Tree Poster Drawings for Kids & Students ✏️✨
-
UP Police Vacancy 2025–26: Applications Open for New SI & ASI Jobs (537 Posts)
-
DigiLocker Alert: Do You Have a Fake DigiLocker App on Your Phone?
-
Not the Gift They Wanted? Why Children Feel Disappointed and How Parents Can Help 🎁💔
-
Phone Cover Safety Alert: Risks of Storing Money and Cards in Smartphone Covers
-
Home Loan Rates Falling: Should You Take a Home Loan Now or Wait for Further Cuts in 2026?
-
Why Vitamin D Deficiency Is a Global Crisis and How to Protect Your Bone Health
-
Taxpayers Alert: Beware of Income Tax Refund Messages
-
Government’s Mega Plan to Prevent Train Accidents (Simplified Explanation)
-
Travel Insurance Mistakes to Avoid: Don’t Let One Small Error Ruin Your Dream International Trip
-
Christmas Style Goals: Take Cues from Bollywood Divas to Slay the Festive Season
-
Do You Really Need Eight Glasses of Water a Day?
-
🇺🇸 Lights On, Curtains Up: Champion Grand USA Premieres Today
-
🎬 The Giant Enters the Arena: Why Dhurandhar 2 Has Everyone Nervous
-
🌍 Lights, Camera, Legacy: Rajamouli Turns His Lens to Varanasi
-
Ananya Panday’s Soft Power Era: When Quiet Fashion Makes the Loudest Statement
-
🌏 From Local Legend to Global Canvas: Rajamouli’s Vision for Varanasi
-
🎄 Merry Christmas Greetings 2025
-
Christmas 2025 Celebrations in Mumbai: Enjoy the Festive Holiday with Unique Regional Food Flavors
-
🎄 Merry Christmas Wishes, Messages, Greetings, Quotes & Image Ideas 🎄
-
🔮 Tarot Horoscope Today – December 25, 2025
-
Wishes :Happy Christmas Day 2025! 🎄✨
-
🎵 ‘Kismat Ki Chaabi’ Song Is OUT NOW!
-
Christmas 2025 Special: Best Places to Visit in Mumbai for a Perfect Festive Experience
-
Merry Christmas 2025: Create Stunning AI Portraits for Instagram, Facebook & WhatsApp 🎄🤳
-
Dhurandhar 2 Date Locked: Too Soon or Perfect Timing?
-
God of War: Puzzle Continues Between NTR & Allu Arjun
-
🎄 Christmas 2025 Quotes, Greetings, Wishes & Bible Verses to Share with Loved Ones ✨
-
📊 Single Ticket Pricing System on Cards in Andhra Pradesh —
-
🎬 Is the Dhurandhar Villain Really Leaving Drishyam 3?
-
🎄 30+ Easy Christmas Tree Drawing Ideas with Santa & Gifts for School Kids 🎅🎁
-
🔔 20+ Easy and Simple Christmas Bell Drawing Ideas for Kids & School Students 🎄✏️
-
Dune vs Doomsday – The Biggest Global Box Office Clash of 2026
-
From Student No. 1 to Champion: The Rise of Jr. NTR
-
🎬 Chiranjeevi Comes With a Thrilling Action Poster!
-
🎬 Beyond the Screen: Cinema Technology and IMAX’s Immersive Experience
-
🎬 Samyuktha’s Next Film Shoot Completed — When Will It Hit Theatres?
-
Science of the Season: The Chemistry Behind Christmas Puddings 🎄🍮
-
🎬 Animal Set for Japan Release on 13 February 2026
-
🎄 25+ Christmas Greetings for Friends, Siblings, Teachers & Parents to Wish Merry Christmas ✨
-
December 25, 2025 horoscope with predictions, lucky numbers and colours
-
Christmas 2025 Special: Unique and Budget Friendly Secret Santa Gift Ideas for Office
-
5 feel good watches on Prime Video this Christmas 2025 —
-
Christmas 2025: Home Decoration Ideas to Make Your Home Look Festive and Welcoming
-
Christmas 2025 Special Recipe: How to Make Perfect Bakery-Style Cookies Without an Oven
-
Christmas 2025 Special Cake Recipe: How to Make a Delicious Chocolate Cake at Home
-
Christmas Celebration in Delhi: Best Places for Parties, Carnivals, and Peaceful Evenings
-
Hailey Bieber’s Viral Vanilla Chai Cupcakes: Easy Step-by-Step Recipe 🍰☕
-
Christmas Travel 2025: Must-Visit Ancient Churches in Bandra ⛪🎄
-
Christmas Travel 2025: Famous Churches to Visit Across India 🎄⛪
-
‘Anaconda’ Review — When Self-Aware Comedy Bites Back and It’s Gloriously Fun
Empowering 140+ Indians within and abroad with entertainment, infotainment, credible, independent, issue based journalism oriented latest updates on politics, movies.
India Herald Group of Publishers P LIMITED is MediaTech division of prestigious Kotii Group of Technological Ventures R&D P LIMITED, Which is core purposed to be empowering 760+ crore people across 230+ countries of this wonderful world.
India Herald Group of Publishers P LIMITED is New Generation Online Media Group, which brings wealthy knowledge of information from PRINT media and Candid yet Fluid presentation from electronic media together into digital media space for our users.
With the help of dedicated journalists team of about 450+ years experience; India Herald Group of Publishers Private LIMITED is the first and only true digital online publishing media groups to have such a dedicated team. Dream of empowering over 1300 million Indians across the world to stay connected with their mother land [from Web, Phone, Tablet and other Smart devices] multiplies India Herald Group of Publishers Private LIMITED team energy to bring the best into all our media initiatives such as https://www.indiaherald.com




 click and follow Indiaherald WhatsApp channel
click and follow Indiaherald WhatsApp channel